Catheterisation Emergencies
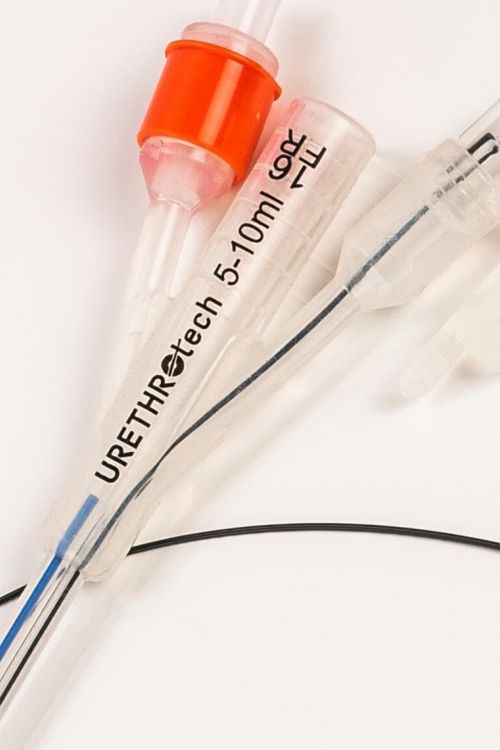
Urethral Catheterisation Device (UCD®)
The UCD® is a simple yet effective catheterisation device designed to solve difficult urethral catheterisation.
Safe & Efficient patient care:
The patented Urethrotech Urethral Catheterisation Device (UCD®) is a breakthrough Urology Device that assists healthcare professionals to solve failed urethral catheterisation and provide value-based care.
A non-traumatic hydrophilic Nitinol guidewire is integrated into a standard urethral 3-way catheter design providing a ready-to-use medical device. The result is successful catheterisation and undisrupted patient care, avoiding the medical emergency of ‘failed urethral catheterisation’.
Difficult male urethral catheterisation is not an infrequent problem and injuries are expensive.
- 0.7/1,000 adult male hospital admissions are affected ²
- 3 – 13.4 /1,000 male catheters inserted lead to urethral injury ² ³
- 4-7% men undergoing cardiovascular surgery are affected ⁴
Traumatic urethral catheterisation injury leads to
- 6% of all urological referrals over 1 year in one teaching hospital ⁵
- Multiple catheterisation attempts cause significant urethral injury in 32% of men ⁶
Urethral Catheterisation Injury is an expensive complication
- £7,285.00 (€8,500.00) per case ⁷
- £287,388.00 (€335,377.00) additional healthcare provider costs over a 6 month study period in two hospitals ⁷
Safe Catheter Insertion
The non-traumatic guide wire forms one unit with the catheter and is inserted into the bladder first, bypassing enlarged prostate lobes.
Universally Suitable
With a 5-year shelf life, the UCD® is suitable for any clinical environment and for every healthcare professional who normally performs urethral catheterisation.
Value Based & Efficient High Quality Care
Avoidance of more invasive specialist procedures and hospital admissions.
- Safe and easy for patient and provider
- Ready-to-use consumable
- Suitable for use by qualified doctors and nurses
- Improved patient care pathway
- Avoid invasive specialist interventions and associated costs
- Complications are reduced, providing safer and better care
- Clinically proven and evaluated by NICE*
*National Institute of Clinical Excellence, United Kingdom
- Purpose-designed and regulated medical device
- FDA 510(k), CE-mark, ISO 13485:2016
- Integrated hydrophilic Nitinol guidewire
- 100% Silicone 3-way Foley Catheter 16F
- The UCD-guidewire is removed after successful catheter placement
- Suitable for bladder irrigation or therapeutic bladder installation
- Attached plug to close off guidewire (irrigation) channel
[1] Urethrotech UCD for difficult or failed catheterisation. Medtech innovation briefing. Published: 7 August 2017. Available from: nice.org.uk/guidance/mib116
[2] Kashefi C, Messer K, Barden R, Sexton C, Parsons JK. Incidence and prevention of iatrogenic urethral injuries. J Urol 2008;179(6):2254–22.
[3] Davis NF, Bhatt NR, MacCraith E, Flood H, Mooney R, Leonard G, Walsh MT. Long-term outcomes of urethral catheterisation injuries: a prospective multi-institutional study. World J Urol. 2020;38(2):473–480.
[4] Bugeja S, Mistry K, Yim IHW, Tamimi A, Roberts N, Mundy AR. A new urethral catheterisation device (UCD) to manage difficult urethral catheterisation.World J Urol. 2019;37(4):595-600.
[5] Thomas AZ, Giri SK, Meagher D, Creagh T. Avoidable iatrogenic complications of urethral catheterisation and inadequate intern training in a tertiary-care teaching hospital. BJU Int 2009; 104(8):1109–1112.
[6] Bacsu C, Van Zyl S, Rourke KF. A prospective analysis of consultation for difficult urinary catheter insertion at tertiary care centres in Northern Alberta. Can Urol Assoc J 2013; 7(9–10):343–347.
[7] Davis NF, Quinlan MR, Bhatt NR, Browne C, MacCraith E, Manecksha R, Walsh MT, Thornhill JA, Mulvin D. Incidence, cost, complications and clinical outcomes of iatrogenic urethral catheterization injuries: a prospective multi-institutional study. J Urol. 2016;196(5):1473-1477.
[8] Guideline for Prevention of Catheter-Associated Urinary Tract Infections (2009). Available from: https://www.cdc.gov/
[9] Kuriyama A, Takada T, Irie H, Sakuraya M, Katayama K, Kawakami D, Iwasaki H, Fowler K, Tokuda Y, Saint S. Prevalence and Appropriateness of Urinary Catheters in Japanese Intensive Care Units: Results From a Multicenter Point Prevalence Study. CID 2017; 64 (2): S127–S130.
[10] Awad MA, Ostenberg EC, Chang H, Gaither TW, Alwaal A, Fox R, Breyer BN. Urethral catheters and medical malpractice: a legal database review from 1965 to 2015. Transl Androl Urol 2016; 5(5):762–773.
[11] Fluckiger S, John H. A new Urethral Catheterisation Device for safe urethral catheterisation in difficult cases. J Clin Urol. 2019;12(83).
[12] Dragova M, Bamfo A, Holmes K, Attard K, Frost A, Mundy A. Managing difficult catheterisation in nurse-led catheterisation services: Does guidewire-assisted urethral catheterisation make a difference? Int J Urol Nurs. 2020;1–7.

Book LIVE-Demo
-
Free live-Demo Sessions with our Expert Trainers
-
Get up to speed in no time!
-
Indications and contraindication for using the UCD-catheter
User Feedback
Real world feedback
Listen to the experience of frontline healthcare professionals using our new product.